Does the Cell Cycle Continue After Death
The Birth and Death of Cells
The Cycle of Growth and Replication
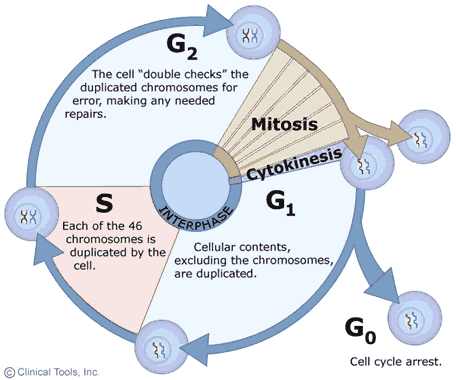 The growth and replication of cells is often described as a cyclic process with two main phases: interphase , when the cell grows and replicates DNA in preparation for cell division, and mitosis , during which the actual division of the cell into two daughter cells occurs. The events occurring in this cyclic process are summarized in the diagram on the right in which interphase events are shown with blue arrows and mitosis is shown in brown. Note that cells may also exit the cycle and enter a G0 phase either temporarily or more or less permanently. In cells that are actively growing and dividing, such as those in an embryo, the cycle is completed frequently as cells divide over and over as the embryo grows and develops. In adults the need for growth and development has passed, and most cells remain in the G0 phase during which they perform their specialized functions, but they no longer replicate (e.g., nerve and muscle cells). Nevertheless, even in fully developed adults certain progenitor cells retain the ability to replicate and give rise to new daughter cells to replace cells that are damaged or lost due to wear and tear. For example, Clara cells in the epithelium of the respiratory tract have the capacity to replicate to produce two daughter cells - one that will be a new Clara cell and one that will differentiate into a replacement epithelial cell. Similarly, hematopoetic stem cells in bone marrow have the ability to replicate to give rise to progenitor cells that can differentiate into the various cellular elements of blood.
The growth and replication of cells is often described as a cyclic process with two main phases: interphase , when the cell grows and replicates DNA in preparation for cell division, and mitosis , during which the actual division of the cell into two daughter cells occurs. The events occurring in this cyclic process are summarized in the diagram on the right in which interphase events are shown with blue arrows and mitosis is shown in brown. Note that cells may also exit the cycle and enter a G0 phase either temporarily or more or less permanently. In cells that are actively growing and dividing, such as those in an embryo, the cycle is completed frequently as cells divide over and over as the embryo grows and develops. In adults the need for growth and development has passed, and most cells remain in the G0 phase during which they perform their specialized functions, but they no longer replicate (e.g., nerve and muscle cells). Nevertheless, even in fully developed adults certain progenitor cells retain the ability to replicate and give rise to new daughter cells to replace cells that are damaged or lost due to wear and tear. For example, Clara cells in the epithelium of the respiratory tract have the capacity to replicate to produce two daughter cells - one that will be a new Clara cell and one that will differentiate into a replacement epithelial cell. Similarly, hematopoetic stem cells in bone marrow have the ability to replicate to give rise to progenitor cells that can differentiate into the various cellular elements of blood.
Regulation of the cell cycle is of critical importance to the aging process. Replication should only occur when there is a need for growth and development (in embryos and the young) or when there is a need to replace damaged or lost cells. Thus, the cycle is influenced by growth factors and by proto-oncogenes that favor replication and by anti-oncogenes that produce proteins that inhibit replication. These various factors interact to regulate the cell cycle in cells that have retained the capacity to divide. In addition, there are cellular processes that constitute checkpoints that prevent the cell cycle from proceeding if errors have occurred. The first checkpoint occurs during the G1 phase and provides an opportunity for cellular that processes to repair damaged DNA before the cell enters the S phase when replication occurs. This prevents the daughter cells from inheriting damaged DNA, which would result in mutations. There are also other checkpoints in S and G2 phases that check for damaged DNA and failure of DNA replication. The final cell cycle checkpoint occurs at the end of mitosis and checks for any chromosomes that have been misaligned. The many factors that regulate the cell cycle play an important rol in the aging process, because as cells age their capacity to replicate diminishes to the point that they are no longer able to divide. As this occurs, the ability to replace damaged or lost cells dwindles and ultimately results in declines in tissue strength and cellular and organ function that are characteristic of aging.
Aging and p53
Protein 53 (p53) is a tumor suppressor protein that is encoded by the TP53 gene. The p53 protein has been extensively studied because the gene that encodes it has been has been found to be mutated in approximately half of all human cancers. Arrest at the G1 checkpoint is mediated by p53 and is rapidly induced in response to damaged DNA. Following the arrest of cell division, p53 will also induce either cell death through apoptosis or permanent loss the ability of a cell to proliferate (senescence). The accumulation of senescent cells contributes to aging because it leads to reduced tissue renewal and repair. Several studies conducted in both mice and humans on mutant p53 genes have supported the notion that increased cancer protection by p53 can also lead to a shortened life life span.
Studies in Mice
Tyner et al studied the effects of p53 mutations in [Nature 2002;415:45-53]. Specifically, they examined the effects of either the absence of p53 or presence of a smaller than normal p53 protein in mice. In order to do this, two transgenic mouse lines were used that had either a shortened form of p53 gene (p53m, partial mutant) or a complete deletion of the p53 gene (p53-, complete deletion). They compared three groups of mice:
- group 1, p53+/p53-(deletion of one copy of p53);
- group 2, p53+/p53m (partial deletion mutant); and
- group 3, p53+/p53+ (wild type, normal).
The table below from Tyner et al. summarizes the results with respect to both cancer formation and aging ,
In group 1 (absence of p53) 80% of the mice developed tumors, while none of the mice in group 2 (shortened p53 gene) developed tumors, and 45% of the mice in group 3 (normal, wild type) developed tumors. Furthermore, group 1 had the shortest life span, group 3 (normal) had the longest life span, and group 2 had an intermediate life span. Thus, partial p53 mutation reduced the risk of developing cancer, but at the cost of a decrease in longevity. The authors also found that the group 2 mice developed phenotypes characteristic of old mice (hunchbacked spines, slow hair re-growth, etc.) earlier than the normal group 3 mice did.
Studies in Humans
Studies by Dumont et al. found that replacement of the amino acid arginine (Arg) with the amino acid proline (Pro) at position 72 on the human p53 protein decreased its ability to initiate cell death and reduced suppression of cancer. A meta-analysis by Heemst, et al. reported that carriers of this Pro/Pro genotype (two alleles with the arginine replaced by proline) have an increased risk of developing cancer compared to Arg/Arg carriers (no replacement of arginine by proline) (p<0.05). Furthermore, they conducted a prospective study of 1226 people aged 85 years or older and found that carriers of the Pro/Pro genotype had a 41% increased survival rate (p = 0.032) despite also having a 2.54 fold increase (p = 0.007) in mortality from cancer. They also found that the Pro/Pro genotype resulted in a significant decrease in mortality from all causes except for cancer and a non-significant increase in mortality from cancer. Overall, they concluded that just as in mice, human p53 protects against cancer but at a cost of longevity. [See Heemst DV, et al.: Variation in the human TP53 gene affects old age survival and cancer mortality. Experimental Gerontology 2005; 40: 11-15
Apoptosis
Apoptosis, also known as programmed cell death, is a regulated process that takes place throughout life and serves to eliminate necessary or damaged cells. Apoptosis takes place during embryonic development as a means of reshaping tissues during normal growth and development, and it provides a mechanism of eliminating worn out or damaged cells throughout life. Age-related diseases such as Alzheimer disease and Parkinson's disease have been linked to an increase in apoptosis where cells that might otherwise continue to support proper tissue functioning are eliminated. There are many pathways and proteins that regulate apoptosis, such as p53, that are able to sense cells that are damaged or no longer needed. The image below illustrates a cell undergoing apoptosis.
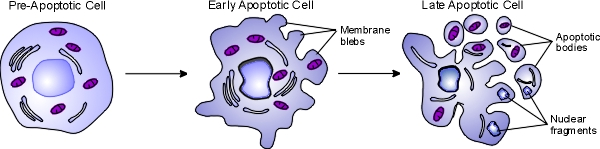
Once molecular signals trigger apoptosis, catabolic processes are initiated in the cell. Various enzymes begin to break down cellular cellular components and fragment nuclear DNA. The chromatin condenses, the cell begins to shrink and irregular bulges in the plasma membrane known as blebs form. The cell eventually breaks into several smaller pieces known as apoptotic bodies containing the cell components and the nucleus. The apoptotic bodies are then taken up by macrophages and removed.
Autophagy
Autophagy is another mechanism by which cell death can occur. Like apoptosis, it is a highly regulated process that plays a normal role in cell growth, development and homeostasis. Autophagy allows a starving cell to reallocate nutrients from unnecessary processes to more essential ones, and it also plays an important housekeeping role by removing misfolded or aggregated proteins, clearing away damaged organelles, such as mitochondria and endoplasmic reticulum, as well as eliminating intracellular pathogens. Some authors believe that mitochondrial dysfunction, which is believed to contribute to cellular aging, occurs in part because of the inability of cells to remove their damaged mitochondria. In addition, autophagy degrades proteins to enable their replacement and also degrades damaged proteins that are no longer functional. Some studies suggest that caloric restriction in rodents increases their life span by preventing the decline in autophagy that occurs with aging.
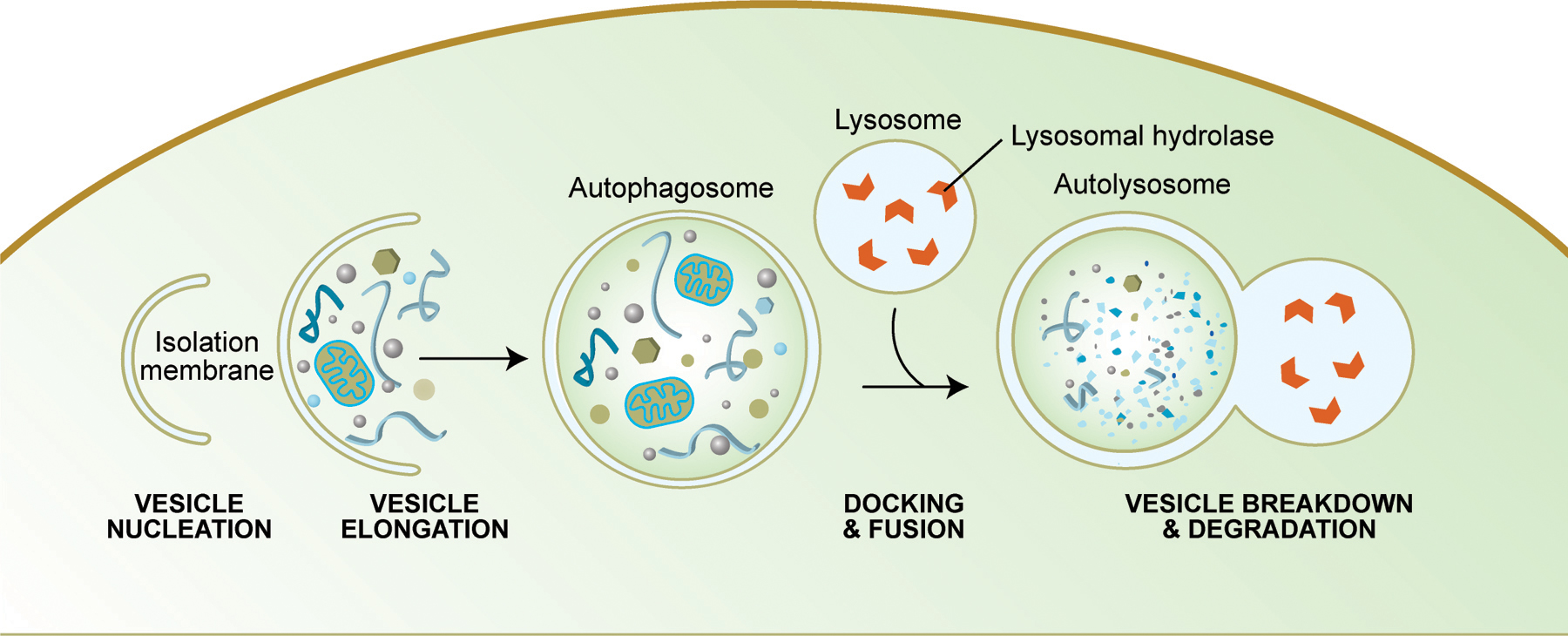
Summary of Autophagy
Source: http://www.wormbook.org/chapters/www_autophagy/autophagy.html
Source: https://sphweb.bumc.bu.edu/otlt/mph-modules/ph/aging/mobile_pages/Aging2.html
0 Response to "Does the Cell Cycle Continue After Death"
Post a Comment