If You Had Tb Once Can You Get It Again
- Research article
- Open Access
- Published:
Risk factors for recurrent tuberculosis after successful handling in a loftier burden setting: a cohort study
BMC Infectious Diseases volume 20, Article number:789 (2020) Cite this article
Abstract
Groundwork
People successfully completing treatment for tuberculosis remain at elevated risk for recurrent disease, either from relapse or reinfection. Identifying risk factors for recurrent tuberculosis may help target mail-tuberculosis screening and care.
Methods
We enrolled 500 patients with smear-positive pulmonary tuberculosis in South Africa and collected baseline data on demographics, clinical presentation and sputum mycobacterial cultures for 24-loci mycobacterial interspersed repetitive unit-variable number tandem repeat (MIRU-VNTR) typing. Nosotros used routinely-nerveless administrative data to identify recurrent episodes of tuberculosis occurring over a median of vi years afterward successful treatment completion.
Results
Of 500 patients initially enrolled, 333 (79%) successfully completed treatment for tuberculosis. During the follow-up menstruation 35 patients with successful handling (eleven%) experienced a bacteriologically confirmed tuberculosis recurrence. In our Cox proportional hazards model, a three+ AFB sputum smear grade was significantly associated with recurrent tuberculosis with a run a risk ratio of 3.33 (95% CI i.44–7.7). The presence of polyclonal Chiliad. tuberculosis infection at baseline had a chance ratio for recurrence of i.96 (95% CI 0.86–iv.48).
Conclusion
Our results indicate that AFB smear grade is independently associated with tuberculosis recurrence after successful treatment for an initial episode while the association between polyclonal M. tuberculosis infection and increased risk of recurrence appears possible.
Introduction
Patients who take recently completed handling for tuberculosis (TB) are at elevated take chances of recurrent TB affliction, either equally a result of relapse or reinfection [1, two]. When individuals are diagnosed with recurrent TB, they are less likely to complete treatment and suffer higher bloodshed than those with first episodes of TB [3]. While in that location is a growing appreciation of the risks associated with recurrent TB, a clearer moving-picture show of which covariates place individuals at greater risk of recurrent illness tin can inform more targeted mail-tuberculosis intendance and monitoring.
Several covariates have been found to be associated with recurrence of TB subsequently completion of handling. Drug-resistance [4], smoking [5], HIV infection with depression CD4 lymphocyte counts [half dozen], substance utilise [7], chronic lung disease [eight], sputum smear-positive disease [9], and cavitary pulmonary illness [10] have each been found to exist associated with increased risk of recurrent tuberculosis. These factors may increase hazard of recurrence because they make it more likely for some modest numbers of mycobacteria to persist beyond treatment or because they are associated with allowed incompetence that places individuals at greater risk of illness following reinfection [11].
Over the last few years, molecular genetic tools have provided a new view on the extent of within-host genotypic heterogeneity of Mycobacterium tuberculosis infections. These "mixed-strain" or "heterogeneous" infections arise either through sporadic mutation (clonal heterogeneity) or host conquering of more than one strain of Grand. tuberculosis (polyclonal heterogeneity), either at the fourth dimension of infection or through series re-infection [12]. While detection of mixed-strain infection is limited by the biological samples collected equally well equally the laboratory processes and sensitivity of sequencing, the frequency at which clonal heterogeneity and polyclonal heterogeneity are reported is nearly xx% in some settings [xiii]. While several studies take institute that within-host heterogeneity, especially polyclonal infections, are associated with poorer treatment response [14], the effects on risk of recurrence are less clear [thirteen].
Studies of recurrent TB are challenging to acquit considering the gamble of recurrent TB can remain elevated for up to 10 years [2]. In this analysis, nosotros combined a inquiry database with electronic notification records to study the long term hazard of recurrent tuberculosis amidst a cohort of ambulatory pulmonary TB patients with a high prevalence of HIV co-infection afterwards successful completion of anti-tubercular therapy.
Methods
Setting
The Umgungundlovu health district, in KwaZulu-Natal (KZN), South Africa had a tuberculosis notification rate of 880 cases per 100,000 during the initial study catamenia of 2011. Local HIV prevalence was xvi.9% and approximately lxx% of people with active tuberculosis were co-infected with HIV [xv].
Participants
All adults with newly diagnosed pulmonary tuberculosis and sputum smear-positive for acrid-fast bacilli (AFB) from five principal health care clinics in the Umgungundlovu wellness commune were eligible for the study. Between June 2011 and November 2012 we enrolled 500 participants and collected two spot pre-treatment sputum samples. Immediately afterwards enrollment, participants were initiated on antitubercular therapy according to South African Department of Health guidelines. Further details of written report blueprint are available in a previous publication [xvi].
Laboratory
Pre-treatment sputum samples were cultured and those positive for M. tuberculosis were genotyped with 24-loci mycobacterial interspersed repetitive unit of measurement-variable number tandem echo (MIRU-VNTR) typing at Genoscreen (Institute Pasteur, Lille, France) [17]. Details of the laboratory procedures have been published previously [sixteen]. For samples with multiple repeats at any MIRU-VNTR loci, indicating more than than genotype, the ClassTR algorithm [18] was used to distinguish clonal M. tuberculosis infection from polyclonal 1000. tuberculosis infection.
Follow-up
Later completion of antitubercular therapy, nosotros tracked outcomes by using routinely nerveless electronic handling annals (ETR) [nineteen] data. The Umgungundlovu health district ETR data includes l clinics serving a population of over one,000,000 people, and also encompasses the original v sites from which participants were recruited [20]. Each facility in the district records tuberculosis case data, including final treatment outcome, in a standardized newspaper tuberculosis register. The health district collects local facility tuberculosis registers and enters them into a commune-wide ETR.
Nosotros matched study participants with commune data in ETR past exact matching of identifiers (e.g. proper noun, gender, date of birth, treatment start date). Every bit there is no unique patient identifier in South Africa and there are several opportunities during administrative data collection for transcription errors, we conducted fuzzy matching for study participants not located by exact matching. We allowed for substitutions of numerical variables and used Jaro-Winkler [21] altitude for matching string variables. A study writer (PC) reviewed all potential matches. We recorded the concluding treatment consequence for each participant. For participants who the ETR listed as lost to follow-up or who transferred their care during treatment, further exact and fuzzy searches were made to endeavor to link intendance episodes and establish a final treatment event.
For report participants with documented treatment success (by Earth Wellness Organization defined [22] cure or handling completion) of their initial episode of tuberculosis, a search for secondary episodes of tuberculosis was then made in the electronic register during the period from initial study enrollment though March 30, 2019. For any secondary tuberculosis episodes found in the electronic register, nosotros recorded the time to recurrence from the initial diagnosis as well as the method of tuberculosis diagnosis (empiric, smear microscopy, Gene Xpert MTB/Rif, or civilisation), and treating dispensary.
Nosotros constructed Cox proportional hazards models with an outcome of recurrent tuberculosis. Participants were censored on the date the ETR search was made. For a multivariable model, nosotros included covariates for inside-host M. tuberculosis heterogeneity (uncomplicated, clonal, and polyclonal) as this was the focus of the written report besides every bit HIV status, given its potent clan with recurrent tuberculosis, and a gaussian frailty random furnishings term for the health center to adjust for clustering. In addition, covariates with a p-value of < 0.20 (age, instruction, marital status, and AFB sputum smear grade) in univariable models were included.
Statistical analysis
R statistical software was used to perform statistical analyses [23]. Cox proportional adventure models were constructed using the packages survival [24] version 2.42–six, survminer [25] version 0.4.3, and prodlim [26] version 2018.04.18 to assess whether any factors were associated with recurrent tuberculosis afterward treatment completion for the initial episode. Proportionality was assessed with correlations between Schoenfeld residuals and log (time) for each covariate and the global model. Multicollinearity was assessed using variance inflation factor.
Results
During the study enrollment and follow-upward period, 110,859 cases of tuberculosis were recorded in the Umgungundlovu Health District ETR. Of the 500 patients initially enrolled, nosotros matched 478 (96%) within the ETR (Fig. 1). 135 (28%) had a handling outcome of "not evaluated" due to clinic transfer or loss to follow-upward. Further searches allowed discovery of the treating clinic of 89 of these 135 (66%) to make up one's mind a final handling effect. 421 (88%) of the matched participants had a positive baseline mycobacterial culture with successful MIRU-VNTR genotyping.
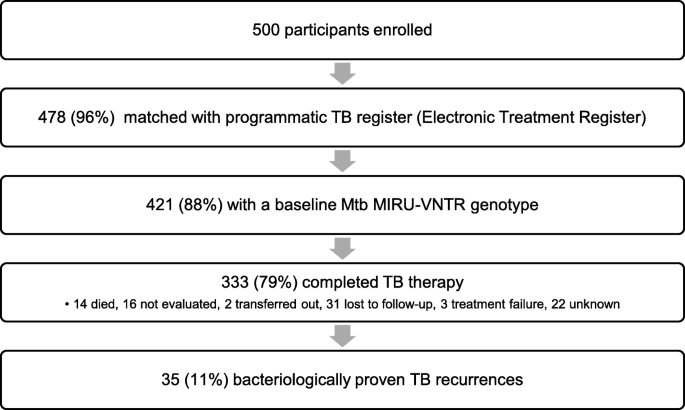
Participant flow diagram
333 (79%) of the original written report participants with a MIRU-VNTR genotype were recorded as achieving treatment completion or cure for their initial tuberculosis episode (Table 1). Those with a successful treatment outcome were less likely to exist living with HIV (65.8 vs 79%) and less likely to have sputum negative for AFB (40.8% vs 56.3%) (Supplemental Tabular array 1). In this cohort, median age was 35 years, and 57% were men. 228 (69%) were HIV positive and 77 (23%) had bear witness of heterogeneous K. tuberculosis infection by MIRU-VNTR genotyping. When utilizing the ClassTR algorithm to interpret MIRU-VNTR results, 30 (9%) had clonally heterogeneous M. tuberculosis illness, and 47 (fourteen%) had polyclonal heterogeneous K. tuberculosis disease. 59 (18%) had a history of tuberculosis prior to enrollment. 7 (2%) had multidrug-resistant TB (MDR-TB).
The time from initial tuberculosis diagnosis to searching in the District ETR was a median of 2125 days (interquartile range 2032 to 2271 days). Fourty-v participants (14%) were diagnosed with recurrent tuberculosis, of which 35 participants (eleven%) had bacteriologically proven tuberculosis, by either sputum smear, Cistron Xpert, or culture, later on a median of 504 days. Kaplan-Meier curves for bacteriologically proven recurrent tuberculosis later treatment for either simple, clonal or polyclonal M. tuberculosis tuberculosis infection were not statistically significantly different by log-rank test (Fig. 2).
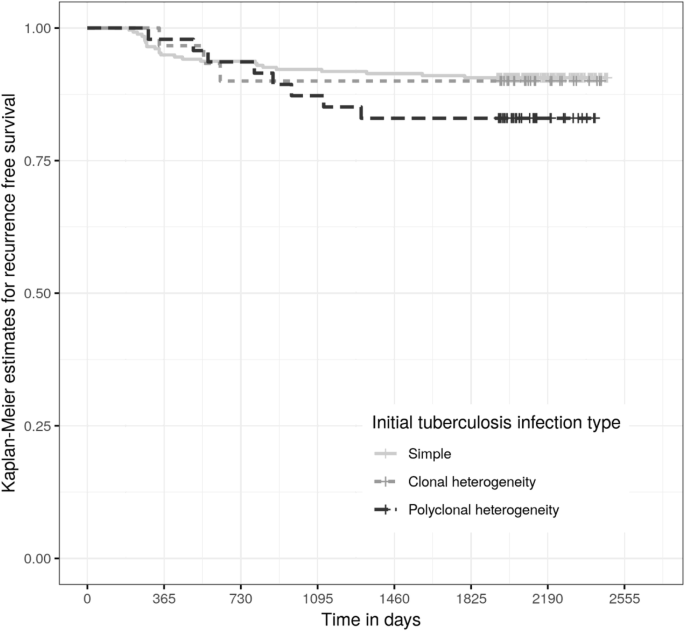
Kaplan Meier estimates for freedom from recurrent tuberculosis after completion or cure
In univariable analysis with Cox proportional hazards models, younger age was significantly associated with increased chance of bacteriologically proven tuberculosis recurrence with a hazard ratio of 0.94 (95% confidence interval (CI) 0.91–0.98) for every additional year of age (Table ii). In our multivariable model, a 3+ AFB sputum smear grade was significantly associated with recurrent tuberculosis with a hazard ratio of 3.33 (95% CI ane.44–seven.vii, p = 0.005) (Fig. 3). The presence of clonally heterogeneous M. tuberculosis infection at baseline had a gamble ratio for recurrence of 0.92 (95% CI 0.27–3.ane), while polyclonal heterogeneous M. tuberculosis infection at baseline had a hazard ratio for recurrence of 1.96 (95% CI 0.86–4.5). None of those with recurrent tuberculosis had MDR-TB at baseline, which fabricated inclusion of MDR-TB in our models impossible.
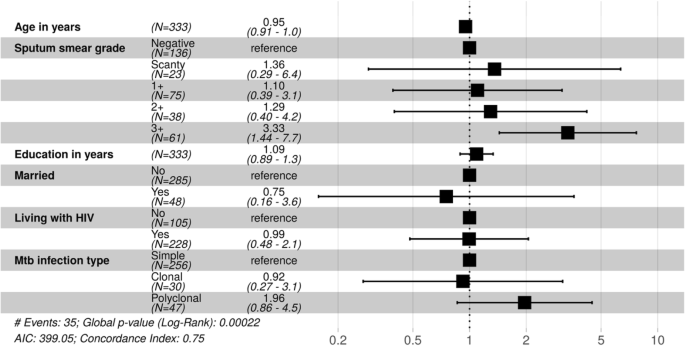
Adjusted hazard ratios for recurrent TB
Discussion
Subsequently following a cohort of largely HIV-coinfected ambulatory individuals successfully treated for pulmonary tuberculosis disease in a high burden setting for a median of vi years, we found that 11% (95% CI 7.2 to 13.8%) had bacteriologically proven recurrent tuberculosis and an additional 3% (95% CI 1.2 to 4.8%) had empirically-diagnosed recurrent tuberculosis. This is consistent with other studies performed in high burden settings [2, 27].
Our finding of an clan between higher sputum AFB smear grade during the initial tuberculosis episode and recurrent episodes of tuberculosis may be due higher mycobacterial load disease and cavitary disease, leading to a higher hazard of relapse later treatment completion. The association between smear form and poor handling outcomes, including recurrent affliction, has been seen in other studies [28].
In this long-term follow-up, the point estimate for the odds of recurrence for individuals with polyclonal Chiliad. tuberculosis infection was 1.96, merely the 95% confidence interval did not exclude i. The relatively pocket-size number of outcomes recorded limits our power to make conclusive statements about whether such polyclonal infections make it more likely for patients to experience relapse after successful handling. An association of recurrent tuberculosis and heterogeneous M. tuberculosis infection is plausible equally heterogeneous infection leads to the potential for heteroresistance. Additionally, Shin and colleagues recently found an association between heterogeneous M. tuberculosis infection and treatment failure in Botswana that was independent of heteroresistance [14]. A high mycobacterial burden inside a host or repeated exposures to tuberculosis in a high TB prevalence environment could be other mechanisms for an association of heterogeneous K. tuberculosis infection and poor treatment outcomes or recurrence.
HIV co-infection was non associated with the risk of recurrence in univariable or multivariable analysis. This could reverberate rise rates of antiretroviral therapy in our setting, and that Due south African guidelines at the time of our initial study recommended placing patients living with HIV and tuberculosis co-infection on antiretrovirals. Antiretrovirals reduce the gamble of developing agile tuberculosis, only how that gamble compares to the HIV uninfected population is not currently known [6, 29, 30]. Alternatively, participants living with HIV could have suffered higher mortality rates in subsequent years that may accept biased our results given nosotros did not have access to vital statistics.
There were limitations in linking our study dataset with ETR data to identify recurrent tuberculosis episodes as there is currently no national health identifier in South Africa. Fuzzy matching of patient identifiers identified many instances of likely matches that were initially missed due to misspellings and transpositions, just we do not know if other matches were missed due to larger discrepancies and whether this introduced bias into our findings. If take chances factors of recurrence are too associated with death, lack of access to vital statistics may have biased results by not properly censoring participants who died during the follow-up period.
This limitation leads to our finding of 11% recurrence as a lower bound on the burden of recurrent tuberculosis. Additionally, even though the ETR encompasses a health district that serves over 1,000,000 people, the population of KZN is highly mobile [31] and the ETR database does not capture recurrences outside of the wellness district. 135 (28%) of the participants in this study transferred clinics or were lost to follow-up during their initial TB handling and we were able to locate 66% of them at their last treating clinic within the wellness commune. Information technology is conceivable that migration out of the health district may have introduced some bias into our study.
Another limitation is that our sampling was at one timepoint from i anatomical source (sputum). Our group's work with mail service-mortem studies in the same population as represented in this report has constitute meaning proportions of multi-organ disseminated tuberculosis with strain heterogeneity that was not captured when sampling sputum alone [32]. Genotyping with MIRU-VNTR as well has limited sensitivity for determining M. tuberculosis strain multifariousness and may have underestimated the true proportion of heterogeneous infection [12]. We also were unable to genotype M. tuberculosis strains from recurrent episodes to distinguish reinfection from relapse. In other studies with matched fingerprinting of initial and recurrent tuberculosis episodes, reinfection accounted for at least half of recurrent disease, reflecting the hyperendemic communities to which people living with tuberculosis return to after treatment completion. We suspect similarly loftier rates of reinfection occurred in our population too, merely we practice not have genotypes from recurrent tuberculosis episodes to test this hypothesis.
Conclusions
Our results indicate that AFB smear grade is independently associated with tuberculosis recurrence after successful treatment for an initial episode while the association betwixt polyclonal 1000. tuberculosis infection and increased take a chance of recurrence appears possible, but further studies are needed to meliorate establish this relationship.
Availability of data and materials
The data sets compiled and analyzed for the current study are available from the corresponding author on reasonable request.
Abbreviations
- AFB:
-
Acrid-fast bacilli
- CI:
-
Confidence interval
- ETR:
-
Electronic treatment register
- KZN:
-
KwaZulu-Natal
- MDR-TB:
-
Multidrug-resistant tuberculosis
- MIRU-VNTR:
-
Mycobacterial interspersed repetitive unit-variable number tandem echo
- TB:
-
Tuberculosis
References
-
Marx FM, Floyd S, Ayles H, Godfrey-Faussett P, Beyers Northward, Cohen T. High burden of prevalent tuberculosis amidst previously treated people in southern Africa suggests potential for targeted control interventions. Eur Respir J. 2016;48(4):1227–30.
-
Uys P, Brand H, Warren R, van der Spuy One thousand, Hoal EG, van Helden PD. The gamble of tuberculosis reinfection before long after cure of a start illness episode is extremely high in a Hyperendemic community. PLoS One. 2015;10(12):e0144487.
-
Murray J, Sonnenberg P, Shearer SC, Godfrey-Faussett P. Human immunodeficiency virus and the outcome of treatment for new and recurrent pulmonary tuberculosis in African patients. Am J Respir Crit Care Med. 1999;159(3):733–forty.
-
Sunday Y, Harley D, Vally H, Sleigh A. Impact of multidrug resistance on tuberculosis recurrence and long-term result in Prc. PLoS One. 2017;12(1): e0168865. https://doi.org/10.1371/periodical.pone.0168865.
-
Yen Y-F, Yen Grand-Y, Lin Y-S, Lin Y-P, Shih H-C, Li L-H, et al. Smoking increases risk of recurrence later successful anti-tuberculosis handling: a population-based study. Int J Tuberc Lung Dis. 2014;eighteen(four):492–8.
-
Golub JE, Durovni B, King BS, Cavalacante SC, Pacheco AG, Moulton LH, et al. Recurrent tuberculosis in HIV-infected patients in Rio de Janeiro. Brazil AIDS Lond Engl. 2008;22(18):2527–33.
-
Kim L, Moonan PK, Heilig CM, Yelk Woodruff RS, Kammerer JS, Haddad MB. Factors associated with recurrent tuberculosis more than than 12 months after treatment completion. Int J Tuberc Lung Dis Off J Int Spousal relationship Tuberc Lung Dis. 2016;twenty(ane):49–56.
-
Pettit AC, Kaltenbach LA, Maruri F, Cummins J, Smith TR, Warkentin JV, et al. Chronic lung illness and HIV infection are take chances factors for recurrent tuberculosis in a low-incidence setting. Int J Tuberc Lung Dis Off J Int Wedlock Tuberc Lung Dis. 2011;15(7):906–11.
-
Pascopella L, DeRiemer K, Watt JP, Flood JM. When Tuberculosis Comes Dorsum: Who Develops Recurrent Tuberculosis in California? PLoS One. 2011;6(11): e26541. https://doi.org/x.1371/journal.pone.0026541.
-
Sonnenberg P, Murray J, Glynn JR, Shearer S, Kambashi B, Godfrey-Faussett P. HIV-one and recurrence, relapse, and reinfection of tuberculosis after cure: a accomplice study in due south African mineworkers. Lancet. 2001;358(9294):1687–93.
-
Mirsaeidi M, Sadikot R. Patients at high risk of tuberculosis recurrence. Int J Mycobacteriology. 2018;7(1):1–6.
-
Cohen T, van Helden PD, Wilson D, Colijn C, McLaughlin MM, Abubakar I, et al. Mixed-strain mycobacterium tuberculosis infections and the implications for tuberculosis handling and control. Clin Microbiol Rev. 2012;25(4):708–19.
-
McIvor A, Koornhof H, Kana BD. Relapse, re-infection and mixed infections in tuberculosis disease. Pathog Dis. 2017;75(3) Bachelor from: https://bookish.oup.com/femspd/article/75/3/ftx020/3003284. [cited 2022 Oct 19].
-
Shin SS, Modongo C, Baik Y, Allender C, Lemmer D, Colman RE, et al. Mixed Mycobacterium tuberculosis–Strain Infections Are Associated With Poor Treatment Outcomes Amid Patients With Newly Diagnosed Tuberculosis, Independent of Pretreatment Heteroresistance. J Infect Dis. 2018; [cited 2022 Oct 19]. https://doi.org/10.1093/infdis/jiy480/5066433.
-
Massyn N, Day C, Dombo Chiliad, Barron P, English R, Padarath A. District health barometer 2012/13 [internet]. Health Systems Trust: Durban; 2013. Available from: http://www.hst.org.za/publications/District%20Health%20Barometers/CompleteDHB2012-2013.pdf.
-
Cohen T, Chindelevitch L, Misra R, Kempner ME, Galea J, Moodley P, et al. Within-host heterogeneity of One thousand. tuberculosis infection is associated with poor early treatment response: a prospective accomplice written report. J Infect Dis. 2016;213(11):1796–ix.
-
Oelemann MC, Diel R, Vatin 5, Haas Westward, Rüsch-Gerdes S, Locht C, et al. Assessment of an optimized mycobacterial interspersed repetitive- unit-variable-number tandem-repeat typing system combined with Spoligotyping for population-based molecular epidemiology studies of tuberculosis. J Clin Microbiol. 2007;45(3):691–vii.
-
Chindelevitch L, Colijn C, Moodley P, Wilson D, Cohen T. ClassTR: classifying within-host heterogeneity based on tandem repeats with application to mycobacterium tuberculosis infections. PLoS Comput Biol. 2022 Feb 1;12(ii):e1004475.
-
ETR.Net - Country Implementations [Net]. 2007 [cited 2022 February 24]. Available from: http://www.etrnet.info/CountryImplementations.aspx.
-
Section of Health. Umgungundlovu Health Commune: Almanac Report 2017/18 [Cyberspace]. 2022 [cited 2022 October 12]. Available from: www.kznhealth.gov.za/2017-2018-Annual-Written report.pdf.
-
Winkler WE, Thibaudeau Y. An Awarding of the Fellegi-Sunter Model of Record Linkage to the 1990 U.Southward. Decennial Census; Technical report, US Agency of the Census; 1987. p. 22. Study No.: RR91–09.
-
World Health Organization. Global Tuberculosis Report 2019. Geneva; 2019. Report No.: WHO/CDS/TB/2019.15.
-
R Core Squad. R: a language and environment for statistical calculating. Vienna: R Foundation for Statistical Computing; 2019. Available from: https://www.R-projection.org.
-
Therneau TM. A package for survival assay in Southward [internet]. 2015. Available from: https://www.CRAN.R-project.org/parcel=survival.
-
Kassambara A, Kosinski M. survminer: Survival Assay and Visualization [Internet]. [cited 2022 Nov 29]. Bachelor from: https://www.rpkgs.datanovia.com/survminer/alphabetize.html.
-
Gerds TA. prodlim: Production-Limit Estimation for Censored Event History Analysis [Internet]. 2022 [cited 2022 Nov xxx]. Available from: https://www.CRAN.R-project.org/package=prodlim.
-
Marx FM, Dunbar R, Enarson DA, Williams BG, Warren RM, van der Spuy GD, et al. The temporal dynamics of relapse and reinfection tuberculosis afterwards successful treatment: a retrospective accomplice written report. Clin Infect Dis. 2014;58(12):1676–83.
-
Imperial MZ, Nahid P, Phillips PPJ, Davies GR, Fielding Chiliad, Hanna D, et al. A patient-level pooled analysis of treatment-shortening regimens for drug-susceptible pulmonary tuberculosis. Nat Med. 2018;24(11):1708.
-
Van Rie A, Westreich D, Sanne I. Tuberculosis in patients receiving antiretroviral treatment: incidence, adventure factors and prevention strategies. J Acquir Immune Defic Syndr 1999. 2011;56(4):349–55.
-
Bock P, Jennings 1000, Vermaak R, Cox H, Meintjes K, Fatti G, et al. Incidence of tuberculosis among HIV positive individuals initiating antiretroviral treatment at higher CD4 counts in the HPTN 071 (PopART) trial in South Africa. J Acquir Immune Defic Syndr. 2018;77(1):93–101.
-
Camlin CS, Snowfall RC, Hosegood V. Gendered patterns of migration in rural South Africa. Popul Space Place. 2014;20(half dozen):528–51..
-
Lieberman TD, Wilson D, Misra R, Xiong LL, Moodley P, Cohen T, et al. Genomic diversity in autopsy samples reveals within-host broadcasting of HIV-associated mycobacterium tuberculosis. Nat Med. 2016;22(12):1470–four.
Acknowledgements
We wish to thank the KwaZulu-Natal Department of Health and its TB Director, Ms. Jacqueline Ngozo for their help with this study. Nosotros also wish to thank the nursing staff and management at the Umgungundlovu District primary healthcare clinics.
Funding
This work was supported by the Fogarty International Center [grant number 1K01TW011194-01A1 to P.Chiliad.T.C] at the National Institutes of Health and a fellowship from the Burroughs Wellcome Fund/American Society of Tropical Medicine and Hygiene Postdoctoral Fellowship in Tropical Infectious Diseases to P.G.T.C. The funders had no role in study blueprint, data collection and analysis, determination to publish, or training of the manuscript.
Author information
Affiliations
Contributions
PGTC, TC and DW conceived of and deigned this written report. PGTC conducted the information analysis. All authors contributed to manuscript grooming and approval of the terminal version.
Corresponding authors
Ethics declarations
Ideals approval and consent to participate
The Biomedical Research Ethics Committee at the University of KwaZulu-Natal and the KwaZulu-Natal Section of Health, also as the Partners Human Research Committee (Boston, Massachusetts), approved the protocol for the original written report. The University of KwaZulu-Natal'due south Biomedical Inquiry Ethics Committee, the KwaZulu-Natal Department of Wellness, and the Yale University Human Investigations Commission approved the additional use of ETR data for this analysis. Written informed consent was obtained from all participants.
Consent for publication
Not applicative.
Competing interests
The authors declare that they have no competing interests.
Boosted information
Publisher's Annotation
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Admission This article is licensed under a Creative Eatables Attribution four.0 International License, which permits use, sharing, adaptation, distribution and reproduction in whatsoever medium or format, as long every bit you lot give appropriate credit to the original author(south) and the source, provide a link to the Artistic Commons licence, and bespeak if changes were fabricated. The images or other third political party cloth in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the fabric. If material is not included in the commodity's Creative Commons licence and your intended employ is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/past/4.0/. The Artistic Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the information.
Reprints and Permissions
About this article
Cite this article
Cudahy, P.Thousand.T., Wilson, D. & Cohen, T. Risk factors for recurrent tuberculosis after successful treatment in a loftier burden setting: a accomplice study. BMC Infect Dis 20, 789 (2020). https://doi.org/10.1186/s12879-020-05515-4
-
Received:
-
Accepted:
-
Published:
-
DOI : https://doi.org/ten.1186/s12879-020-05515-iv
Keywords
- Mixed-infection
- South Africa
- Relapse
- Reinfection
- Fuzzy match
Source: https://bmcinfectdis.biomedcentral.com/articles/10.1186/s12879-020-05515-4
0 Response to "If You Had Tb Once Can You Get It Again"
Post a Comment